Abstract
Background: Polycythemia vera (PV) and essential thrombocythemia (ET) are associated with an increased risk of thrombosis, progression to myelofibrosis (MF) and significant symptom burden. While hydroxyurea, interferon, and JAK2 inhibitors are used to treat these myeloproliferative neoplasms (MPNs), hematopoietic stem cell-depleting therapies may result in an alternative mechanism to alter disease course and improve outcome. MDM2, a negative regulator of P53, is overexpressed in CD34+ MPN cells harboring wild type P53. It is the target of idasanutlin (IDA), an oral MDM2 antagonist that results in up-regulation of P53 and down-stream activators of apoptosis. Preclinical data from our laboratory group supports the clinical evaluation of IDA in patients (pts) with ET/PV (Lu et al Blood. 2014;124(5):771-9). We evaluated the safety and tolerability of IDA in a phase I dose escalation trial of pts with JAK2V617F-positive ET or PV [NCT02407080].
Methods: 13 pts (1 withdrew prior to treatment) resistant/intolerant to hydroxyurea and/or interferon therapy and without prior JAK2 inhibitor therapy were enrolled in a single institution phase I trial of oral IDA at two dose levels of 100 mg and 150 mg daily for five consecutive days and repeated every 28 day cycles. The first cycle consisted of 56 days for the evaluation of dose limiting toxicities (DLT). A DLT was defined as any non-hematologic AE of grade 3+ or a hematologic AE of grade 2+ thrombocytopenia, or grade 3+ neutropenia or anemia. The absence of DLT in the initial cohort of 100 mg daily in 3 evaluable pts allowed for dose escalation in a new cohort to 150 mg and after a total of 6 pts were treated at this level an additional 3 pts were treated at 100mg to complete the intended study. Pts that did not attain at least a partial response (PR) by modified European LeukemiaNet (ELN) criteria after cycle 6 and met eligibility were able to continue receiving IDA in combination with pegylated interferon-α (PEG) at 45ug weekly. Correlative studies included baseline mutational profiling, MDM2 protein levels, changes in serum MIC-1 levels and JAK2V617F variant allele frequency (VAF) with therapy.
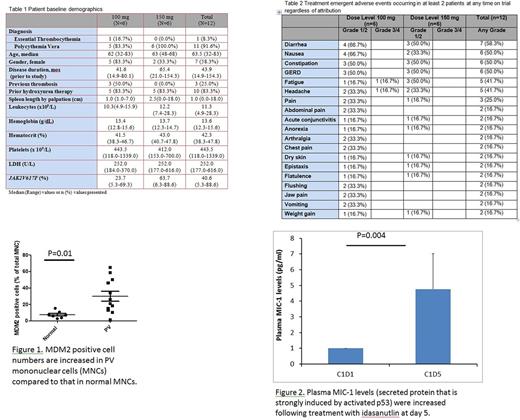
Results: 12 pts (6 at 100 mg; 6 at 150 mg) were treated and their baseline characteristics are shown in Table 1. Pts received a median of 7 cycles (range, 1-12) over a median of 33 weeks (range, 8-107) on study. Three pts discontinued treatment due to pt decision (n=2) and physician decision (n=1). A DLT was not identified for either dose level during cycle 1, and no hematologic treatment emergent adverse events (TEAE) were seen at any time point. Table 2 shows the non-hematologic TEAEs that occurred in at least 2 pts regardless of attribution. Grade 3 fatigue (n=1) and grade 3 headache (n=1) were the only significant TEAE noted at any time on study (both in 100mg cohort). The overall response rate by modified ELN criteria by cycle 7 for the 9 evaluable pts was 7/9 (78%). 7 of 10 (70%) evaluable pts achieved a ≥50% improvement in total symptom score (TSS) from baseline. There were no thrombotic or major hemorrhagic episodes, or progression to MF. Bone marrow pathologic response was assessed in 2 pts after 5 cycles. One case showed histological improvement, with normalization of overall marrow cellularity and megakaryocyte number, and less pronounced megakaryocytic atypia. The median JAK2V617F VAF at baseline, 7 cycles, and 9 cycles of therapy was 45% (n=12), 12% (n=6) and 13% (n=6), respectively. One pt was eligible to receive combination therapy and achieved phlebotomy freedom, normalization of palpable spleen (baseline 18cm), leukocyte count (baseline 44 x109/L), and PV-related symptoms by cycle 8. This pt also attained a 20% reduction in JAK2V617F VAF.
The percentage of mononuclear cells expressing MDM2 was dramatically increased as compared to normal controls (p=0.01) (Figure 1). Serum levels of MIC-1 was used to assess pharmacodynamic effects of IDA therapy. Serum MIC-1 levels were elevated (~ 5 fold) after 5 days of administration of IDA in 85% of the pts (Figure 2), indicating activation of P53 by the low doses of IDA administered. Persistent elevation in MIC-1 levels was observed on day 15 in 6 of the 12 pts treated.
Conclusion: IDA is well tolerated and demonstrates a clear signal of clinical activity in pts with therapy refractory PV. Correlatives support on-target activity. Mature follow up on the entire cohort will be presented. A multicenter phase II trial of IDA at a dose of 150 mg is underway
Mascarenhas: CTI Biopharma: Research Funding; Janssen: Research Funding; Promedior: Research Funding; Novartis: Other: DSMB member , Research Funding; Merck: Research Funding; Incyte: Other: Clinical Trial Steering Committee , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal